
An American company has invented a new piece of technology called the SkinGun, which hopes to help those who have suffered serious burns.
Burn victims can sometimes wait months for skin grafting treatment, but it's hoped this new technology will reduce that rapidly. RenovaCare says the product looks to address America's $45 billion (£34 billion) wound and burn treatment market.
The SkinGun manages to grow a healthy layer of skin over the affected area using stem cells. Check out the slick advertising video here:
The product works by harvesting a small (roughly the size of a postage stamp) patch of skin from your body, the stem cells are separated, put in a solution and sprayed on the wound. The SkinGun uses RenovaCare's other device, the CellMist System, to help suspend the stem cells in the liquid solution.
It takes about 90 minutes for the treatment from start to finish.

Credit: RenovaCare
RenovaCare says it has treated more than 60 patients with the SkinGunn during trials. You can see in the image below how the product has helped victims of chemical and gas explosions, gasoline and electrical burns and hot tar and hot water scalds.
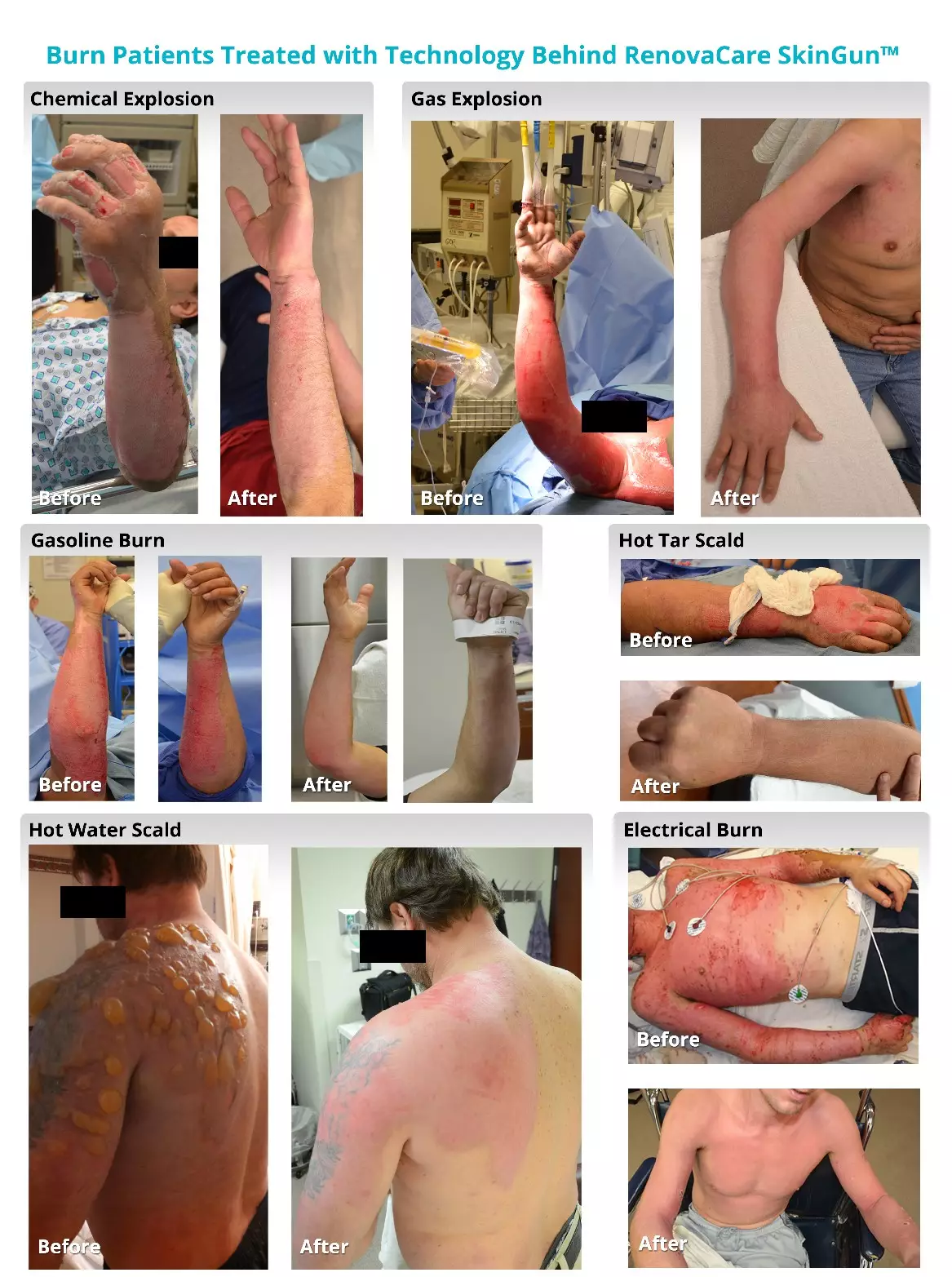
Credit: RenovaCare
In a press release, RenovaCare Chief Executive, Thomas Bold, says: "In the case of one patient with severe electrical burns to over one-third of his body, his wounds were sprayed with 23 million stem cells isolated from a tiny 2" x 2" sample of his own skin. Within five days of treatment, his chest and arms were already healed. Four days later, the patient was discharged from the hospital."
It's important to note that at the end of the video above, a disclaimer reads: "The outcomes described here are from preclinical studies and single case observations from work conducted under Innovative Practice Approach guidelines with Institutional Review Board approval.
"This treatment was provided with a prototype version of the device...the company has announced plans to conduct FDA-regulated clinical studies in the future. There is no assurance that such studies will be successfully undertaken."
So the world has to wait a while before these treatments will be ready for standard use. The company hopes once it has a license in the US it can branch out to Europe.
Featured Image Credit: RenovaCare