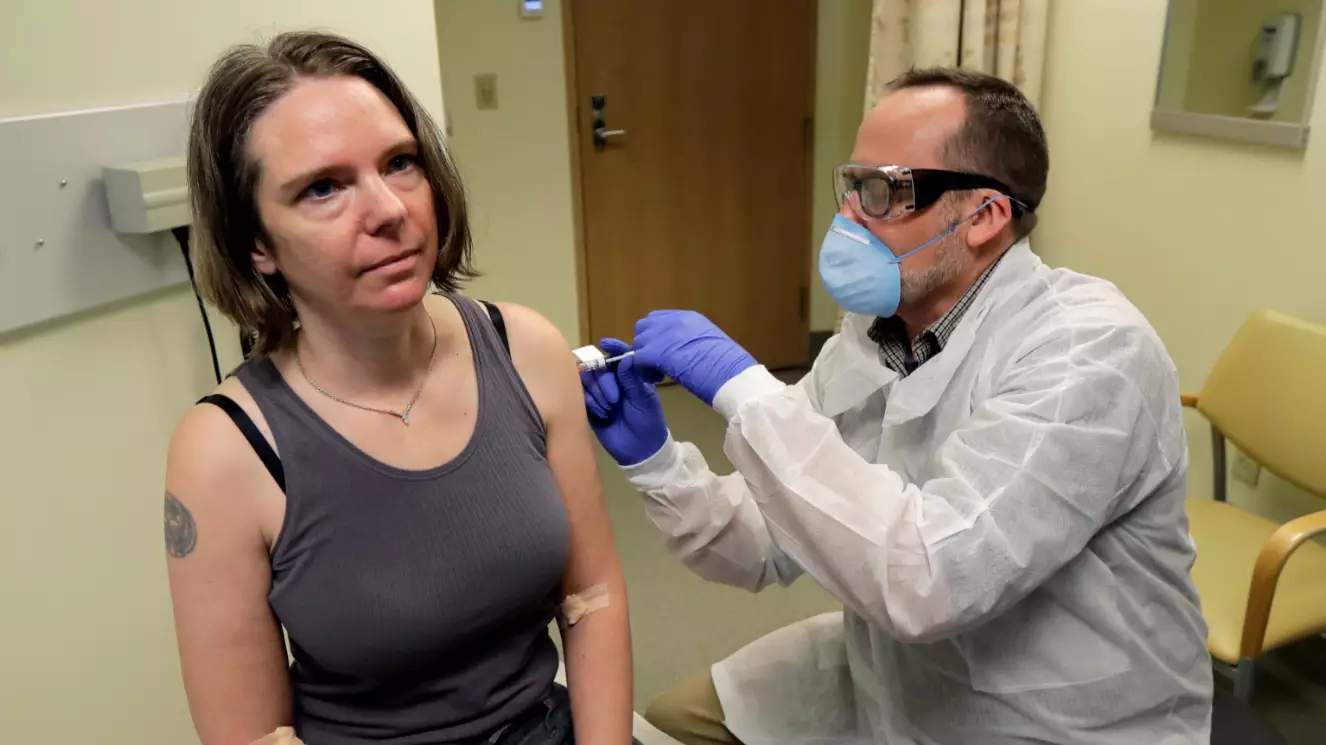
US researchers have now administered the first shots of an experimental coronavirus vaccine, having begun the human trial yesterday (Monday 16 March).
The early-stage trial is being led by Dr Lisa Jackson, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle, Washington, which has become the epicentre of the US outbreak, with 42 of the country's 69 deaths.
Advert
Mum-of-two Jennifer Haller, 43, was the first to receive the shot, admitting her two teenagers 'think it's cool' she's taking part in the study.

Haller said as she awaited her turn: "We all feel so helpless. This is an amazing opportunity for me to do something."
Study leader Jackson said: "Everyone wants to do what they can in this emergency."
Advert
She added: "We're team coronavirus now."
Study volunteers - healthy people aged between 18 and 55 - have been carefully selected for the trial, which will not only test the safety of the vaccine, but also its ability to induce an immune response in the volunteers.
Some people will be getting higher dosages than others to test how strong the inoculations might need to be.
Researchers will be drawing blood samples to see whether or not the vaccine is helping the immune system, and will also be looking out for any side effects.
Advert
"We don't know whether this vaccine will induce an immune response, or whether it will be safe. That´s why we´re doing a trial," Jackson said.
"It´s not at the stage where it would be possible or prudent to give it to the general population."

Over the course of the next six weeks, the team will enrol 45 participants for the trial.
Advert
Jackson continued: "This work is critical to national efforts to respond to the threat of this emerging virus.
"We are prepared to conduct this important trial because of our experience as an NIH clinical trials centre since 2007."
National Institute of Allergy and Infectious Diseases allowed the new vaccine to be fast-tracked into clinical trials.
New drugs must pass through three phases of clinical trials to be deemed safe.
Advert
Director Anthony S. Fauci said in a statement: "Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority."
He added: "This Phase 1 study, launched in record speed, is an important first step toward achieving that goal."
It's okay to not panic. LADbible and UNILAD's aim with our campaign, Cutting Through, is to provide our community with facts and stories from the people who are either qualified to comment or have experienced first-hand the situation we're facing. For more information from the World Health Organisation on Coronavirus, click here.
Topics: World News, News, Coronavirus, US News