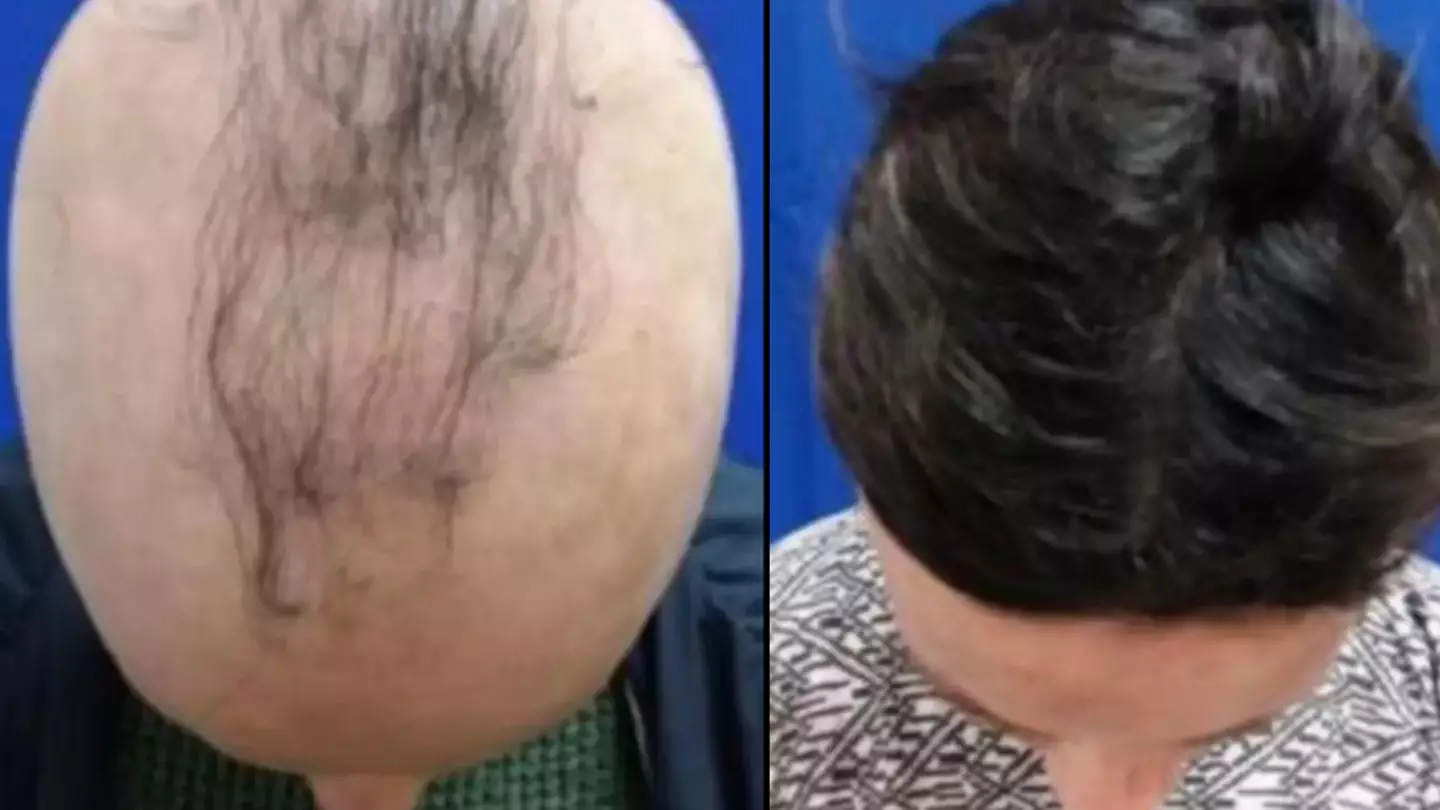
Researchers in the US have discovered a drug that could counter alopecia areta, a type of hair loss caused by the body attacking its own healthy hair follicles.
American pharmaceutical company Concert Pharmaceuticals is currently trialling a twice-daily pill that can combat and reverse rapid hair loss for the condition, which has no cure.
So far, the trial has found that four in 10 participants were able to regrow 80 percent or more of their hair within a year.
The new therapy works by dampening the immune response, preventing it from going haywire.
Advert
This week, the company behind the drug released the discovery of its phase three trials, which looked at 706 adults with alopecia aged 18 to 65 in the US, Canada and Europe over 24 weeks.
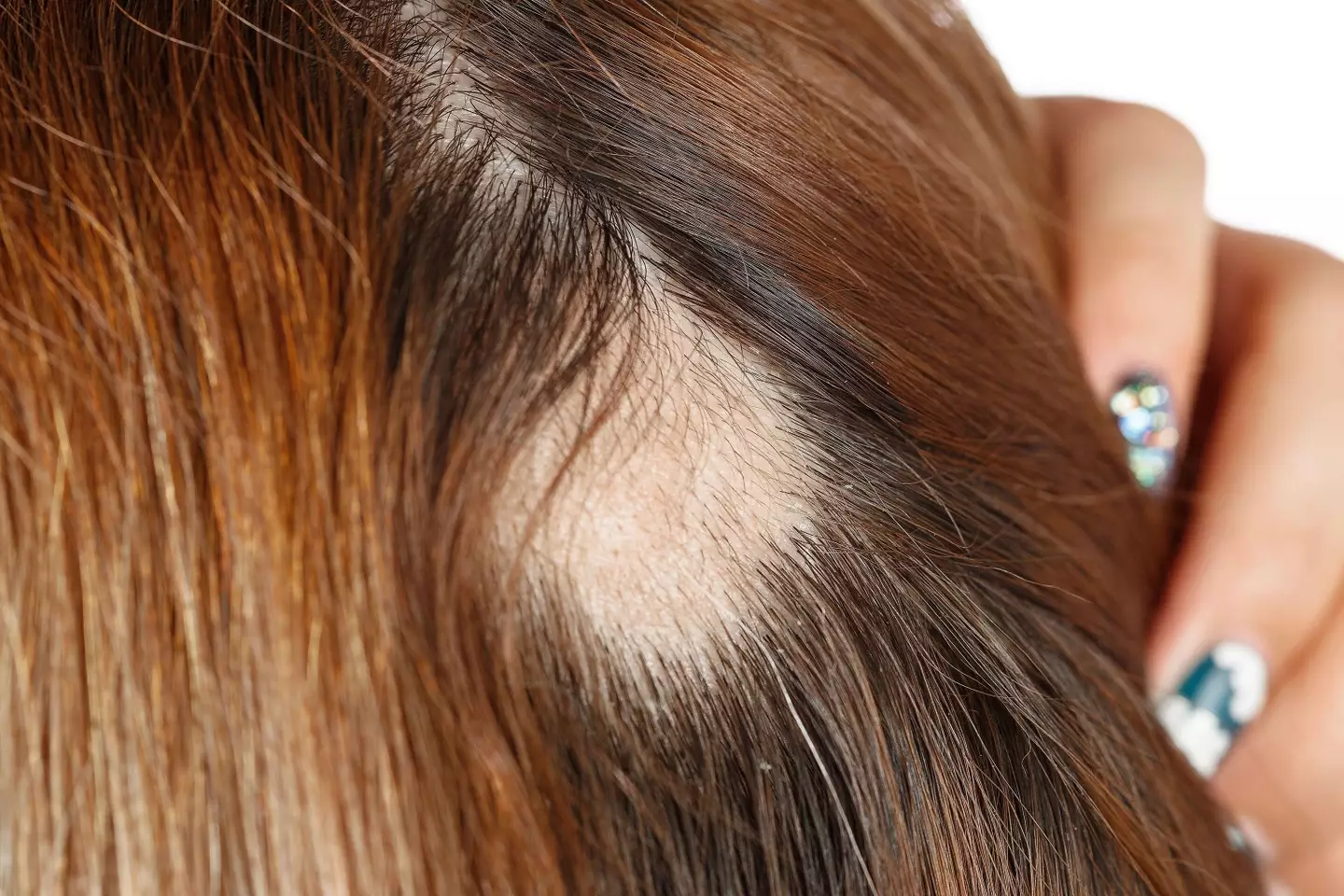
Patients on average had just 16 percent of their hair at the start of trial, and no one had more than 50 percent of hair.
They were split into three groups: one test group were given a placebo, while another an 8mg twice-daily dose, and lastly a 12mg, twice-daily pill, to the third.
By the end, a total of 41.5 percent of those given the strongest does, 12mg of the drug, saw more than 80 percent of their hair regrow while those taking the lower dose had almost 30 percent experienced the same amount of hair regrowth.
In the placebo group, only 0.8 percent of the participants saw more than 80 percent of hair growth.
The drug works by inhibiting a particular enzyme that is activated during an immune response, called JAK1 and JAK2, which make up a group called janus kinases, and too much of them can lead to inflammatory immune responses that cause alopecia.

However, suppressing them can also lead to weaker immunity across the board, resulting in more infections.
Interestingly, researchers said the drug was 'generally well-tolerated', with less than five percent of patients complaining of side effects such as headaches, acne and infections.
Concert Pharmaceuticals is set to undergo another round of phase three clinical trials for this treatment before applying for US Food and Drug Administration (FDA) approval, which could take up to 10 months.
Speaking of the findings, Dr James Casella, chief developmental officer at Concert Pharmaceuticals, said: “With these compelling Phase 3 data, we believe that CTP-543 has the potential to be a best-in-class treatment for patients with alopecia areata, a disease that has long been ignored.
“We are extremely grateful to the patients and teams of clinical research professionals who participate in our trials.
“We’re working to change the treatment landscape and hope that CTP-543 will be one of the first FDA-approved treatment options for this serious disease.”
