
Topics: UK News, News, World News, Health

Topics: UK News, News, World News, Health
The UK Health Security Agency (UKHSA) has issued advice following the spread of a deadly virus with no cure and no vaccine.
India has officially reported two cases of the bat-borne pathogen spreading in hospitals in the eastern state of West Bengal.
So far, the confirmed Nipah virus cases have been reported at the private Narayana Multispeciality Hospital in Barasat, West Bengal, 16 miles from the state capital Kolkata.
With a mortality rate of 40 to 75 percent, symptoms typically include 'fever, headaches, myalgia (muscle pain), vomiting and sore throat'.
Advert
This is often followed by 'dizziness, drowsiness, altered consciousness, and neurological signs that indicate acute encephalitis'.
"Some people can also experience atypical pneumonia and severe respiratory problems, including acute respiratory distress," the World Health Organisation (WHO) says.
"Encephalitis and seizures occur in severe cases, progressing to coma within 24 to 48 hours."
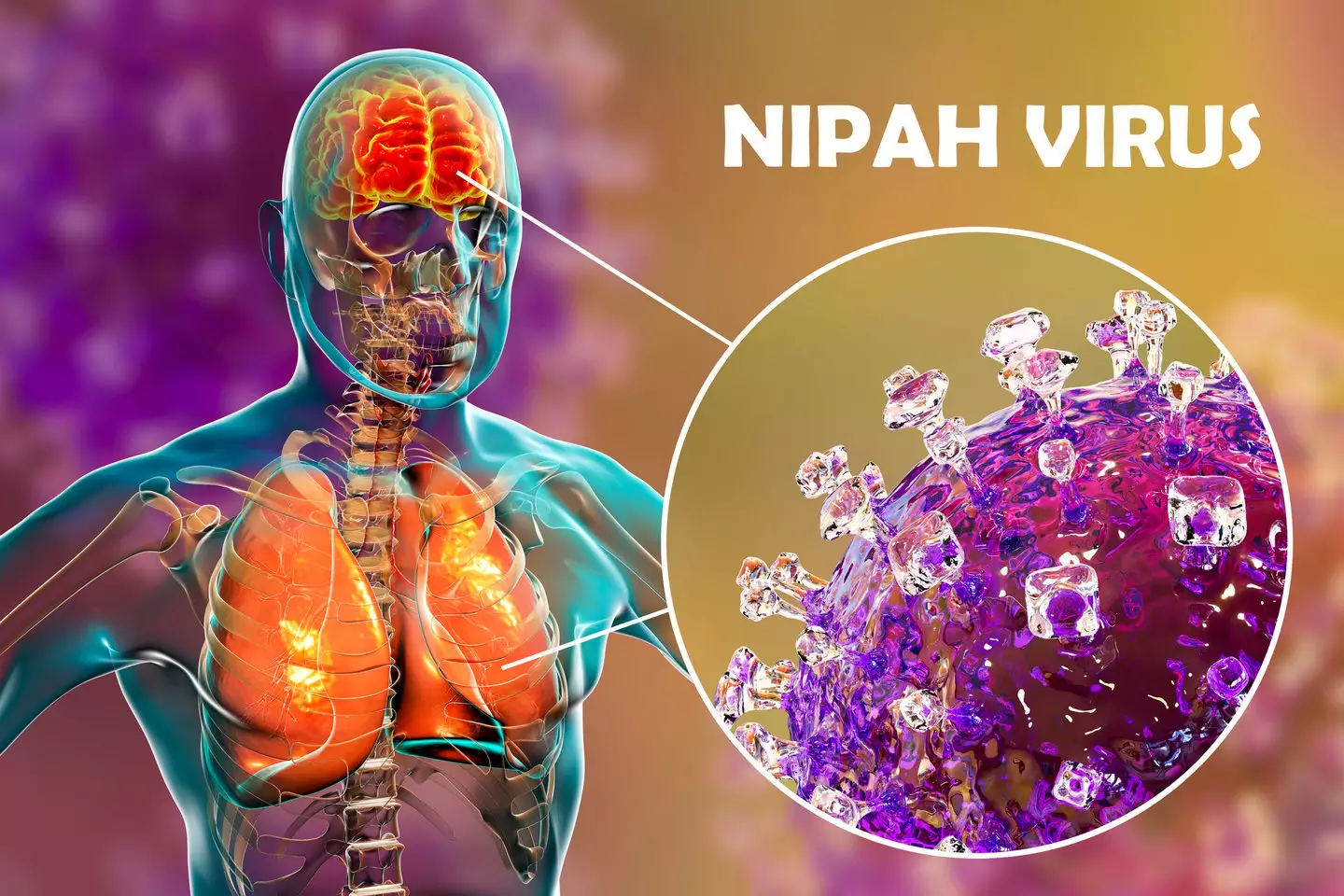
The UKHSA considers Nipah virus a 'high priority' pathogen 'because of its high fatality rate and the fact that there are currently no approved vaccines or specific treatments available'.
Although there has yet to have been a case in the UK, it said that travellers should be wary of going to the affected areas.
"The risk for tourists visiting endemic countries is very low if standard precautions are followed. The risk may be higher for those participating in local practices such as date palm sap collection and consumption," the UKHSA said.
"If you develop symptoms while overseas in an endemic area, seek advice from a health professional immediately.
"If you develop symptoms after returning to the UK, contact your healthcare provider and make sure to mention your recent travel history."
It comes after Professor Brian Angus, Chief Investigator of the Nipah vaccine trial at the Oxford Vaccine Group, tells LADbible that trials are now underway for their 'Nipah vaccine candidate'.
"Following a review of our early CEPI-funded phase 1 clinical trial data, our Nipah vaccine candidate has been granted support from the PRIority MEdicines (PRIME) scheme offered by Europe’s medicines regulator, the European Medicines Agency (EMA)," Professor Angus explained.

"Phase 2 trials are currently underway in Bangladesh, also funded by CEPI [the Coalition for Epidemic Preparedness Innovations], and we hope the data generated in this trial will continue to support the path to licensure for this vaccine."
He warned that the virus is 'highly transmissible' and has the 'potential to cause serious epidemics or pandemics'.
"There are currently no specific medications or licensed vaccines to protect against or treat a Nipah virus infection, and so a future vaccine candidate is a crucial step forward in pandemic preparedness," he added.
Nipah virus was first identified around 1998 during a severe outbreak in Malaysia and Singapore, where pigs acted as the intermediate host.
Bangladesh has experienced almost annual outbreaks since 2001, mainly due to consumption of contaminated date palm sap.
India has reported repeated outbreaks in West Bengal in 2001 and 2007, then in Kerala from 2018, with several fatalities. Sporadic cases have also occurred in the Philippines.
In total, 754 human cases have been reported with 435 deaths globally, according to 2024 stats.
Bangladesh has recorded 341 cases and 241 deaths - a fatality rate of 71 percent.